
Please let us know how we can improve this web app. calcium hydroxide + carbon dioxide = calcium carbonate + waterĮxamples of the chemical equations reagents (a complete equation will be suggested):.PhCH 3 + KMnO 4 + H 2SO 4 = PhCOOH + K 2SO 4 + MnSO 4 + H 2O.To enter an electron into a chemical equation use + H 2O.Compare: Co - cobalt and CO - carbon monoxide Always use the upper case for the first character in the element name and the lower case for the second character.Ğxamples: Fe, Au, Co, Br, C, O, N, F.Enter an equation of a chemical reaction and click 'Balance'.

If so, you’ve successfully written a net ionic equation.Instructions on balancing chemical equations: Those are called “spectator ions.” Check your work by making sure that the total charge on the reactant side is equal to the total charge on the product side. Finally, cross out any ions that appear exactly identical on both sides of the equation. Rewrite the whole equation with each dissociated compound written out this way. After all the atoms are balanced, you have a chemical equation: CH 4 (g) + 2O 2 (g) CO 2 (g) + 2H 2 O (g) 4. Multiplying the coefficient of O 2 by 2 gives 2O 2, which has 4 oxygen atoms. The single Cr atom has 3 positive ions to balance out the negative ions of the 3 Cl atoms. There are now 2 oxygen atoms on the left side (O 2) and 4 on the right side (2H 2 O and CO 2 ). For example, 2CrCl3 would dissociate into Cr3+ + 3Cl. Metals will become positive cations, while non-metals will dissociate into negative anions. Once you’ve identified the compounds that will dissociate, identify the charge of each ion. For instance, a compound that is made up of an oxide or hydroxide and an alkali or alkaline earth metal will completely dissociate in solution. Then, determine which compounds in your equation will dissociate, or separate into positive and negative components, called cations and anions. Write the state as aq, s, or g in parentheses after each compound. For instance, a compound that’s described as “in solution” is aqueous. The Net Ionic Equation demonstrate the part of a reaction that is the. Next, make note of the states of matter of each compound-are they aqueous (or liquid), solid, or gas? If you’re doing a word problem, look for keywords that explain the different states. 4) Balance polyatomic ions as whole units rather than trying to balance the. When you’re done, re-count all of the atoms on each side to make sure they’re equal.
#BALANCED NET IONIC EQUATION CALCULATOR FREE#
The balanced equation will look like C+CO2 → 2CO. The balancing equations calculator with steps divides the redox reaction into half of the reaction. Free math problem solver answers your algebra, geometry, trigonometry, calculus, and statistics homework questions with step-by-step explanations, just like a math tutor. Since there are 2 carbon atoms and 2 oxygen atoms on the reactant side, you’ll need to add the coefficient 2 on the product side. For example, say you want to balance the equation C+CO2 → CO. Start with all of the atoms that aren’t hydrogen or oxygen, then balance the hydrogen and oxygen atoms. Write the number of atoms in the compounds on each side of the equation, then add coefficients in front of the atoms on each side until they’re equal.
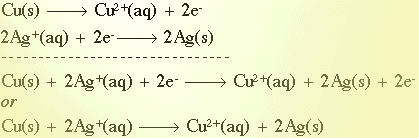
To write a net ionic equation, you’ll need to start by balancing the equation. Rewritten, this equation becomes: 2Cr ( s) + 3NiCl 2( aq) -> 2CrCl 3( aq) + 3Ni ( s).

This is how the redox equations are balanced.
Now, the equation is balanced with 2 Chloride’s (Cl) with total charge -2 and 3 Chromium’s with total charge +3 on both sides. NiCl 2 and CrCl 3 are soluble ionic compounds, therefore, they are aqueous. To balance the unbalanced chloride molecule charges, we add 2 in front of the chloride on L.H.S. Cr and Ni in their elemental forms are solids. For example, 2Cr + 3NiCl 2 -> 2CrCl 3 + 3Ni.If the problem mentions an acid or a base, they will be aqueous ( aq).If there is not water, the ionic compound is a solid ( s).If it has high solubility, the compound will be aqueous ( aq), if it has low solubility, it will be solid ( s). If there is water in the equation, determine whether or not the ionic compound will dissolve using a solubility table.If a compound is said to be a solution, you can write it as aqueous, or ( aq).If no state is provided for an element, use the state found on the periodic table.There are some rules to help you determine the state of an element or compound. Oftentimes, you will be able to identify keywords in a problem that will tell you the state of matter for each compound. Identify the states of matter of each compound in the equation.


 0 kommentar(er)
0 kommentar(er)
